LABGENI TFF ultrafiltration membrane cassettes package
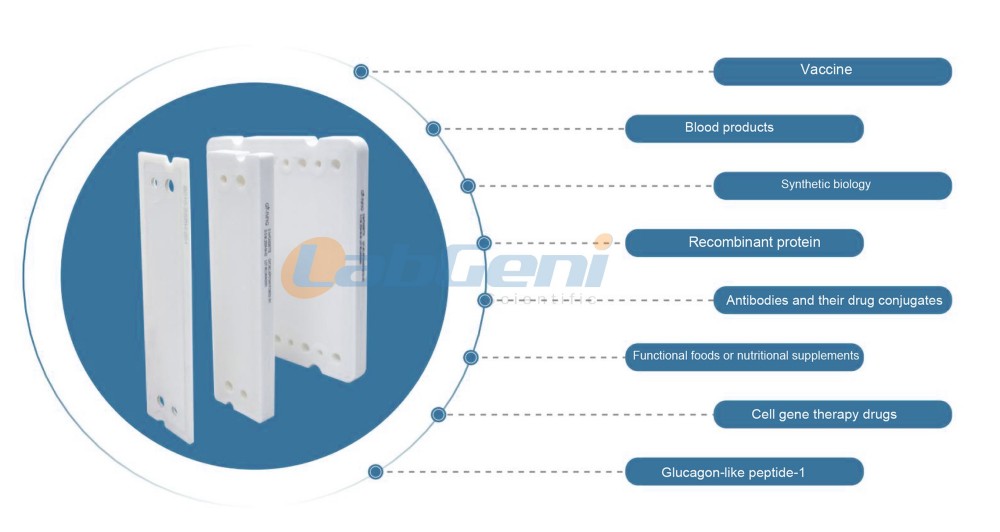
Ultrafiltration membrane cassettes package are a typical tangential flow membrane separation technology solution, widely used in pharmaceutical processes, particularly in downstream purification of biopharmaceuticals, including solution concentration, diafiltration, desalination, and separation and purification of components of varying molecular sizes.

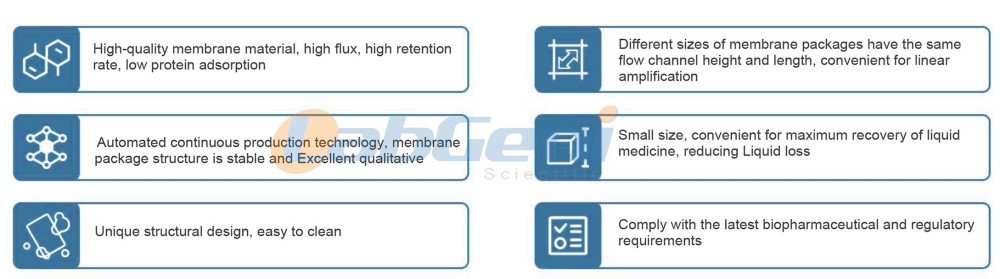
LABGENI BIOTFFC membrane cassettes utilize advanced automated packaging processes and an optimized, dead-angle-free structure. They offer a robust structure and are easy to rinse. They utilize internationally renowned polyethersulfone membranes, which exhibit excellent flux and retention performance, low protein adsorption, and a wide temperature and pH range, making them particularly suitable for biopharmaceutical processes.
LABGENI offers ultrafiltration membrane cassettes with molecular weight cutoffs ranging from 1 to 1000 kD, depending on the membrane’s filtration accuracy.
LABGENI offers six sizes of TFF membrane cassettes, all with identical flow channel lengths and heights, facilitating linear scale-up and suitable for laboratory, pilot, pilot, and mass production stages.
Typical application areas
Advantages OF ultrafiltration membrane cassettes package
Adapter membrane closure
Compatible with mainstream membrane clamps on the market, LABGENI can also provide clamps that meet the requirements.

TechnicalSpecifications
- Material composition
| Filter membrane material | Polyethersulfone |
| Support materials | Polyethersulfone |
| Sieve | Polypropylene |
| Packaging materials | Silicone |
| Gasket | Silicone |
- Regulatory Information
| Biosafety | Complies with USP<87>, USP<88>class VI standards |
| bacterial endotoxins | Comply with <0.25EU/ml |
| Insoluble particles | Compliant with USP<788> standards |
| Quality System | The R&D and production processes are in compliance with ISO9001 and
ISO13485 quality management system |
- Traceability: Each membrane package is serially numbered for full traceability.Includes: A quality assurance certificate, two silicone gaskets, and a user manual, material safety data sheet, and validation guideavailable upon request.
- Packaging and storage: Vacuum-packed in aluminum foil bags. PES membrane packages contain 0.2N sodium hydroxide and 25% glycerol.
Size parameters
| Membrane package size | Application |
| 0.02m² | R&D,laboratory |
| 0.1m² | R&D,laboratory,pilot |
| 0.5m² | Pilot production and mass production |
| 1.0m² | Pilot production and mass production |
| 2.5m² | Pilot production and mass production |
| 5.0m² | Pilot production and mass production |
Ordering Information
| GU2 | E | 010 | F | 010 | P | -X(if any) |
| Series Name | Membrane Type |
Molecular weight cut-off |
Runner type | Effective membrane area |
Applicatio n level |
Customized |
| GU2=Series 2 | E=PES | 001=1KD | F=fine | 002=0.02m² | P=bio-process | |
| @Ultra | V=PVDF | 002=2KD | C=coarse | 010=0.1m2 | F=food | |
| fitration | C=RC | 003=3KD | V=suspended | 050=0.5m² | W=water | |
| 005=5KD | 100=1.0m² | |||||
| 008=8KD | 250=2.5m² | |||||
| 010=10KD | 500=5.0m² | |||||
| 020=20KD | ||||||
| 030=30KD | ||||||
| 050=50KD | ||||||
| 100=100KD | ||||||
| 300=300KD | ||||||
| 500=500KD | ||||||
| 750=750KD | ||||||
| 1000=1000KD |











