In drug development, DSC is often used in the drug screening and optimization process, especially when developing compounds that need to bind to specific proteins. This method is able to measure the thermal behavior of proteins when heated, especially the heat released or absorbed when their structure changes. DSC can provide important information about protein folding and stability, which is very valuable in fields such as drug design, biotechnology and disease research. By comparing the DSC curves of proteins without and with drug binding, it is possible to evaluate how drug candidates affect the thermal stability of proteins.
In the biopharmaceutical field, DSC is often used as a quality control method to ensure the stability and integrity of collagen drugs throughout the production and storage process.
The DSC method can not only provide information on the thermal stability of proteins, but also help study protein folding dynamics and secondary structure. This is achieved by analyzing the different transitions on the DSC curve, which may correspond to different protein structures or other reactions.
1. Experimental Instruments and Settings
1.DSC3000C Differential Scanning Calorimeter
- High purity nitrogen
- Test sample collagen
- Standard aluminum crucible
- Standard tweezers

- Experimental Principles and Steps
- Principle
DSC is a technique that measures the amount of heat absorbed or released by a sample during heating or cooling. By comparing the difference in heat flow between a sample and a reference under temperature changes, the thermal properties of the sample can be obtained. Denaturation of collagen due to heating is called thermal denaturation. Denaturation will change physical, chemical and biological properties. The various biological functions of proteins in life activities are entirely dependent on their specific conformations. Once this specific conformation is destroyed, its biological function will disappear. Therefore, it is of great significance to study the thermal denaturation of collagen under different conditions. Differential scanning calorimetry can directly give the temperature and energy changes of the protein thermal denaturation process, and is a very effective method to study the conformational changes and structural stability of collagen.
- Operation steps
2.1 Take 10-15 mg of sample and put it into a crucible and weigh it.
2.2 Install and adjust the gas flow according to the instrument’s operating manual.
2.3 Prepare a heating program and conduct a heating test in the range of 70-90° at a heating rate of 5°C/min.
2.4 Record experimental data and analyze the results.

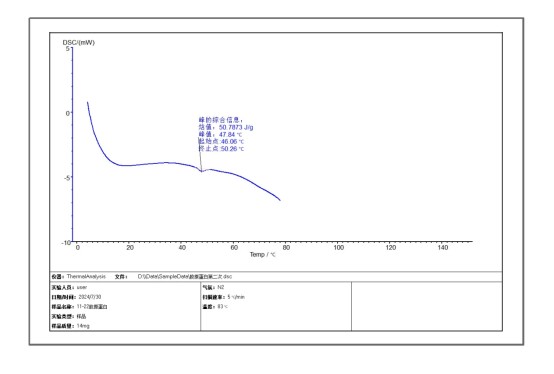
From the experimental analysis of the spectrum, it can be directly seen that the melting point (TM) of the protein is 46°C and a temperature point at which denaturation begins, that is, the stability is destroyed. The thermal enthalpy value is 50J/g. For proteins, this means the total amount of heat used to fold the protein, representing an endothermic process. Since the protein is exposed to elevated temperatures in the DSC experiment, the protein begins to undergo thermal denaturation, accompanied by the breaking of non-covalent bonds.
This experiment can also be used to determine whether a protein is inactivated. In the DSC technique, ΔHcat is determined solely by the area of the integrated DSC thermal transition peak curve, while ΔHvn is determined solely by the shape of the thermal transition peak curve. The sharper the transition peak, the larger the △HvH, and vice versa. Using △Hca/△Hvn can help us estimate whether a large portion of the protein is inactive. If △Hcal is significantly lower than △HvH, it may indicate that a large part of the protein has been inactivated.
In summary, the analysis of DSC data can help us understand the denaturation temperature of the protein and determine whether the protein is inactivated.
